Introduction
Nechamandra alternifolia (Roxb.) Thwaites, a member of the Hydrocharitaceae family, is an aquatic plant species native to Southeast Asia (Liu et al, 2019a). N. alternifolia is an obligate submerged aquatic plant species (APHA, 2017). This species is particularly found in countries such as India, Thailand, Vietnam, and China (Liu et al, 2019b). N. alternifolia var angustifolia has become an essential plant in the aquarium trade due to its ornamental value and ability to oxygenate the water (Raja et al, 2015).
In terms of ecological significance, N. alternifolia plays a vital role in maintaining water quality by absorbing excess nutrients and releasing oxygen into the water column (Brix, 1997). Furthermore, it provides habitat and shelter for several aquatic organisms, including fish and invertebrates, thereby promoting biodiversity within aquatic ecosystems (Cowx, 2002). However, like many other aquatic plants, N. alternifolia can also become invasive when introduced outside its natural range, causing negative impacts on local flora and fauna (Geng et al, 2018). Therefore, proper management strategies should be implemented to prevent the spread of this species beyond its native distribution.
Monotypic Enigmatic Status of N. alternifolia in the Aquatic Family Hydrocharitaceae
N. alternifolia, a submerged aquatic plant of the family Hydrocharitaceae, stands as a monotypic genus with a remarkable ecological profile. Despite its status as the sole species within the genus Nechamandra, this plant exhibits characteristics that set it apart from other aquatic plants, particularly its rapid growth and adaptability to a wide range of aquatic environments.
The species' high degree of endemism in its natural habitat of Asia especially, vast Indian sub-continent, contrasts with its extraordinary capacity for colonization. N. alternifolia thrives in both stagnant and moving waters, displaying an ability to inhabit a diverse range of aquatic conditions, from shallow waters to great depths (Kapoor, 1986). This adaptability, coupled with its fast growth rate, underscores the plant's potential to become invasive under favourable conditions. Such traits are uncommon in monotypic genera, making N. alternifolia an intriguing subject for studies in plant ecology and invasiveness.
The plant’s capacity to colonize various aquatic environments indicates a level of ecological plasticity that might contribute to its survival and proliferation beyond its native range. As N. alternifolia spreads rapidly in different water bodies, it can outcompete native aquatic flora, potentially disrupting local ecosystems (Cook, 1996). This invasive potential adds another layer of complexity to its monotypic status, suggesting that the genus may be more dynamic and ecologically impactful than initially assumed.
Moreover, the species’ ability to colonize both stagnant and flowing waters, as well as varying depths, points to a sophisticated adaptation strategy. This trait enables N. alternifolia to exploit a wide range of ecological niches, making it a formidable species in its habitat. The plant's rapid growth and ability to form dense mats on water surfaces can significantly alter aquatic environments, impacting water quality, light penetration, and oxygen levels, thereby affecting the overall health of the ecosystem (Hussain et al, 2010).The monotypic and enigmatic status of N. alternifolia highlights the dual nature of this species—highly specialized yet potentially invasive. Further research into its ecological strategies and invasion potential is essential for understanding the broader implications of its presence in diverse aquatic environments.
Genetic and Phylogenetic Status of N. alternifolia
N. alternifolia is a species within the Hydrocharitaceae family, exhibiting a unique genetic and phylogenetic profile among aquatic plants. The genetic diversity of this species has been relatively underexplored, but preliminary studies suggest that it possesses a distinctive genetic makeup that separates it from closely related taxa.
Genetic Status
The genetic structure of N. alternifolia is characterized by a low level of genetic diversity within populations, likely due to its clonal mode of reproduction and limited seed dispersal mechanisms (Rao, 2016). Genetic studies utilizing molecular markers, such as RAPD (Random Amplified Polymorphic DNA) and ISSR (Inter Simple Sequence Repeats), have demonstrated that populations of N. alternifolia exhibit high genetic similarity, indicative of a restricted gene flow among populations (Jaiswal & Srivastava, 2015). This genetic homogeneity may increase the species' vulnerability to environmental changes and habitat fragmentation.
Phylogenetic Status
Phylogenetically, Nechamandra alternifolia holds a unique position within the Hydrocharitaceae family, distinguishing itself from other members of the family (example: H. verticillata(L.f.) Royle and Ottelia alismoides (L.) Pers.) through its distinct evolutionary lineage. This differentiation highlights its potential significance in understanding the evolutionary history, adaptive mechanisms, and ecological roles of aquatic macrophytes within this family (Les et al, 2006). Phylogenetic analyses based on chloroplast DNA sequences (e.g., rbcL and matK) have placed Nechamandra in a monotypic genus, suggesting that it diverged early from other Hydrocharitaceae members (Les et al, 2008). The monotypic nature of the genus and its distinct phylogenetic position emphasize the evolutionary significance of N. alternifolia within the family.
Historical Perspective
In the past challenges were faced in identifying and controlling another aquatic weed, H. verticillata, whose widespread introduction caused extensive damage worldwide (Madsen et al, 2015). Misidentifications occurred frequently due to its high morphological similarities with other aquatic plants, leading to difficulties in tracking its dispersal and implementing effective mitigation measures (Madsen et al, 2015).
Current Knowledge Gap
Likewise, N. alternifolia folia faces comparable concerns since its subtle differences compared to other coexisting aquatic plants complicate positive identification (Hussner et al, 2013). As a result, delineating precise microstructural attributes through light microscopic anatomy is imperative in averting imminent threats posed by the potentially aggressive expansion of this variety.
Conservation Implications
Given its unique genetic and phylogenetic status, N. alternifolia is of considerable conservation concern due endemicity. The low genetic diversity within populations, combined with its limited distribution, makes it susceptible to extinction risks undermining its potential as a weed in newer ecosystems. Conservation strategies should prioritize the protection of its aquatic habitats and promote genetic studies to better understand its evolutionary potential and adaptability.
Paradox of N. alternifolia
Considering these attributes, N. alternifolia presents a paradox: while it remains highly endemic to specific Asian regions, its ecological traits position it as a potential invasive species with significant environmental implications for the new worlds. Growing online aquarium trade, growing export-import of aquatic products (like Fish, aquatic plants) has high potential for introducing this species to newer environments. Understanding the factors driving its rapid growth and adaptability is crucial for managing its impact on non-native ecosystems.
Objects and methods of research
Objective
Given the lack of any existing literature on the histology or microscopic anatomy of N. alternifolia, conducting a study on this topic would provide valuable insights and contribute significantly to our understanding of this aquatic plant species. Despite being widely distributed and utilized in various fields such as aquaculture and horticulture, detailed knowledge about its internal structure remains scarce.
Previous research primarily revolves around its macroscopic aspects, while little emphasis has been placed on its microscopic anatomy. Investigating the latter becomes crucial, considering the risks associated with its unfamiliar invasive behavior in foreign waters. This paper underscores the necessity of exploring the light microscopic anatomy of wet N. alternifolia, drawing examples from the well-studied case of H. verticillata and Najas Indica (Willd.) Cham. & Schltdl.
To address this gap, we undertook a comprehensive investigation focusing on the microscopic anatomy of N. alternifolia was undertaken utilizing modern techniques such as light microscopy. By analyzing these tissues, we gained insight into cellular morphology, ultrastructure, and chemical composition.
Additionally, studying the microscopic anatomy of N. alternifolia in comparison with closely related species within the same family Hydrocharitaceae, provided clarity for improved taxonomic classification and identification. Understanding the intricate details of this aquatic plant's microstructure also aided in determining its functional traits and adaptability under varying environmental and stressful conditions.
Morphology of N. alternifolia
N. alternifolia is an aquatic plant species belonging to the family Hydrocharitaceae. It is predominantly found in freshwater habitats in Southeast Asia, including India, Sri Lanka, and Thailand. The morphology of this species is characterized by its distinct vegetative and reproductive structures.
Vegetative Morphology
The plant is a submerged aquatic herb, with slender, flexible stems that can grow up to several meters in length. The stems are typically unbranched or sparsely branched and bear leaves in an alternate arrangement (Kundu et al, 2018). The leaves are linear, measuring about 1-2 cm in length and 1-2 mm in width, and have an entire margin. The leaf blades are thin, translucent, and typically have a single prominent midrib (Cook, 1996).
Reproductive Morphology
N. alternifolia reproduces both sexually and vegetatively. The flowers are unisexual, with male and female flowers occurring on the same plant (monoecious). Male flowers are small, numerous, and borne on short pedicels, while female flowers are solitary and are typically found at the nodes. The male flowers release pollen directly into the water, where it drifts to fertilize the female flowers (Kundu et al, 2018). The fruits are cylindrical, containing numerous seeds that are dispersed by water currents.
Materials and Methods
For this study, fresh samples of N. alternifolia were collected from local water bodies of surrounding Shayadri hills, where this species is endemic. Direct wet mounts were utilized to examine the plant's structure due to its highly primitive and almost unicellular architecture (Bellinger et al, 2019). The samples were then prepared for observation using a compound light microscope with a photography system. Direct wet mounts were utilized to examine the plant's structure due to its highly primitive and almost unicellular architecture (Bellinger et al, 2019). This method allowed us to observe the living cells without the need for staining or other preparation techniques that could potentially alter the natural state of the cells. The choice of this aquatic macrophyte was due to its relatively simple and primitive structure, which allowed for the preparation of direct wet mounts (Gessner, 1957; Sculthorpe, 2003).
To create the wet mounts, a small piece of the plant was placed on a glass slide and covered with a coverslip. A drop of distilled water was added between the slide and coverslip to keep the sample hydrated during observation. Care was taken to avoid trapping air bubbles under the coverslip as they can interfere with the clarity of the image. Careful attention was given to ensure that the depth of field and focus were optimized for each image captured. This method allowed us to observe the living cells directly without the need for staining or other preparation techniques that could potentially alter the natural state of the cells.
The slides were observed and photographed using a Nikon EclipseTM Ni-U compound light microscope equipped with a DS-Fi3 camera system. The microscope was set up with brightfield illumination at magnifications ranging from 40x to 200x. This specialized microscope was available in our human anatomy department for the purpose of studying human histology. Images were captured using NIS Elements software and adjusted for contrast and brightness to enhance visualization of the cell structures. To minimize potential artifacts caused by uneven lighting conditions, Koehler illumination was employed throughout the investigation (Köhler, 1893).
Results and discussion
Gross Histology of the Leaf of N. alternifolia
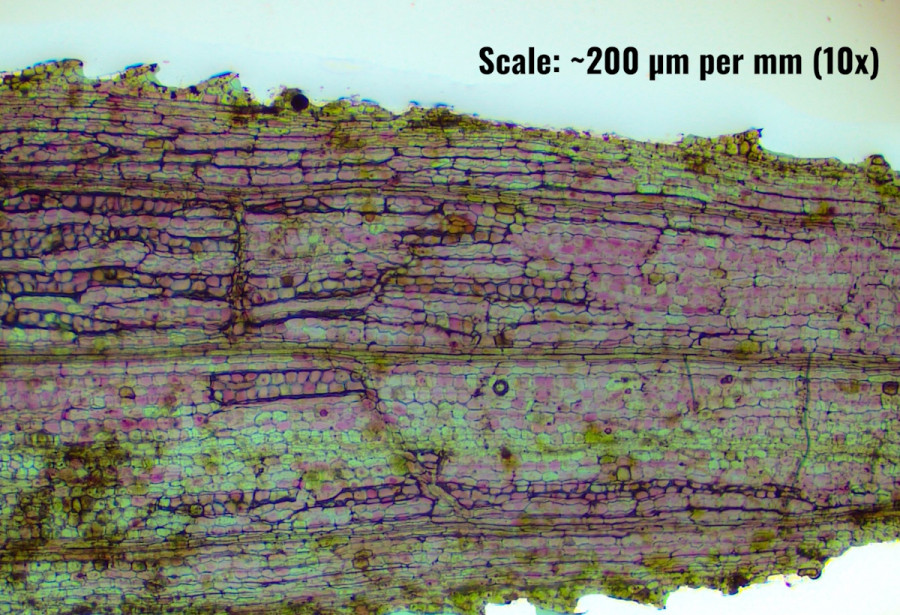
The photomicrograph of the N. alternifolia leaf section at 40x, shown in Fig.1 reveals several key histological features characteristic of aquatic plants, particularly those with a highly thalloid structure. These observations contribute to our understanding of how the leaf is structurally adapted to its submerged environment.
Highly Thalloid Structure
The leaf exhibits a thalloid structure, which is typical of many aquatic plants. This structure is characterized by a flattened, undifferentiated form, lacking the complexity seen in the leaves of terrestrial plants. The thalloid nature suggests a primitive organization with minimal differentiation between tissues, allowing for effective light absorption and gas exchange in an aquatic environment.
Absence of Cuticle
A notable feature of the leaf is the absence of a distinct cuticle layer on the epidermis. In terrestrial plants, the cuticle serves as a protective barrier to prevent water loss; however, in N. alternifolia, which is fully submerged, the absence of a cuticle is consistent with its aquatic lifestyle. This adaptation allows for direct interaction with the surrounding water column, facilitating efficient gas exchange and nutrient uptake across the leaf surface.
Lack of Aerenchyma (Air Spaces) or Sclerenchyma
The section reveals a dense cellular arrangement with no significant aerenchyma or large intercellular air spaces. While many aquatic plants develop aerenchyma to aid in buoyancy and oxygen transport, N. alternifolia appears to rely on a more compact cellular structure. This could be an adaptation to the specific hydrodynamic conditions of its habitat, where buoyancy may be less critical, or to the efficient utilization of space for photosynthetic cells.
Primitive Marginal Teeth (D)
Marginal teeth are present along the edges of the leaf, though they are relatively primitive in structure. These structures might serve various functions, including stabilizing the leaf in water currents or increasing the surface area for light absorption and gas exchange. The presence of these teeth suggests an evolutionary adaptation that enhances the plant's interaction with its aquatic environment.
Chloroplasts and Pigmentation (E)
Chloroplasts are abundantly distributed within the mesophyll cells, indicating the leaf's primary role in photosynthesis. The staining reveals a predominance of chlorophyll, as well as other pigments, possibly carotenoids or anthocyanins, which may contribute to photoprotection. The distribution of chloroplasts throughout the leaf suggests an adaptation to maximize light capture in the underwater environment, where light intensity and quality can vary with depth and water clarity.
Vascular Tissue (F)
The vascular system is minimally developed, with no prominent vascular bundles visible in this section. This reduced vascularization is typical of many aquatic plants, where the need for long-distance transport of water and nutrients is diminished due to the surrounding aqueous medium. The minimal vascular tissue observed in N. alternifolia supports the notion that the plant relies on direct diffusion of nutrients and gases through its tissues, a common adaptation among submerged plants.
Cellular Organization (G)
The cells in Nechamandra leaves are hexagonal and tightly packed, forming a uniform, robust structure. This arrangement helps in maintaining the structural integrity of the leaf and maximizing the photosynthetic area. The mesophyll cells are arranged in a layered manner but lack the clear differentiation into palisade and spongy mesophyll typically seen in terrestrial plants. This homogeneity in cell structure may be an adaptation to the even distribution of light in the aquatic environment, where leaves are often oriented in various directions relative to the light source.
Pigmentation and Staining Patterns (H)
The staining pattern suggests the presence of various pigments within the leaf tissues. In addition to chlorophyll, which is vital for photosynthesis, the presence of other pigments such as anthocyanins or carotenoids may provide additional photoprotection against the fluctuating light conditions underwater.The leaf features pink pigmented cells, which enhance the absorption of blue wavelengths crucial for photosynthesis in open water environments. The observed coloration in the photomicrograph reflects these adaptations, with a mix of pigments contributing to the overall efficiency of the photosynthetic process in the aquatic habitat.
The histological features observed in the N. alternifolia leaf section underscore its specialization for a submerged lifestyle. The absence of a cuticle, the presence of a thalloid structure, the minimal development of vascular tissues, and the uniform distribution of chloroplasts all highlight the plant's evolutionary adaptations to optimize its survival and functionality in an aquatic environment.
Observation of Leaf Marginal Teeth in N. alternifolia at 200x
The photomicrograph (Fig.2) at 200x provides a detailed close-up view of the marginal teeth of the N. alternifolia leaf. This specific focus on the marginal structures reveals several intriguing features that suggest functional adaptations and evolutionary significance:
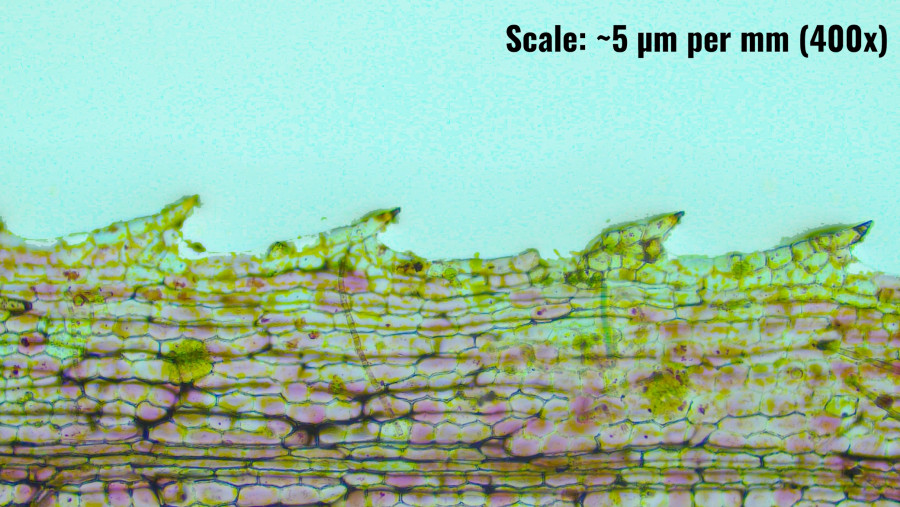
Structure of Marginal Teeth (marked A)
The marginal teeth are prominent and exhibit a pointed, somewhat triangular shape. These structures appear to be composed of several layers of cells, with the cells near the tips being smaller and more compact. This cellular arrangement could indicate a degree of mechanical strength at the edges, which may help the leaf maintain structural integrity against water currents or other environmental forces within its aquatic habitat.
Cellular Composition
The cells forming the marginal teeth appear to be elongated and tightly packed, especially near the tips. The absence of specialized cells like trichomes suggests that these marginal teeth are purely structural rather than defensive. The high cell density might play a role in preventing the tearing of the leaf margin, which is particularly important in a submerged environment where physical stability is crucial.
Functional Implications
The primitive nature of the marginal teeth suggests an evolutionary adaptation that enhances the plant's interaction with its aquatic environment. These structures could play a role in stabilizing the leaf in moving water, reducing the risk of physical damage, or even increasing the surface area for gas exchange and light absorption. The pointed shape of the teeth might also aid in deflecting debris or other particles, thereby maintaining the leaf's cleanliness and efficiency in photosynthesis.
Pigmentation and Staining
The photomicrograph shows that the marginal teeth are similarly stained as the rest of the leaf tissue, indicating that they contain similar cellular components, such as chloroplasts. This suggests that even these edge structures may contribute to the photosynthetic process, albeit to a lesser extent than the broader leaf lamina. The presence of pigments within the marginal teeth cells could also provide some level of photoprotection.
The close-up observation of the marginal teeth of N. alternifolia reveals a structurally significant feature that likely contributes to the plant's overall adaptability in its aquatic environment. The pointed, tightly packed cells provide mechanical strength, potentially preventing damage in flowing water and contributing to the leaf's overall structural stability. The magnification of around 40x to 200x allowed for detailed visualization of these important adaptations, offering insights into the evolutionary strategies employed by this species to thrive in its submerged habitat.
Observation of Leaf Section in N. alternifolia (Highest Magnification)
The high-magnification 200x photomicrograph of the N. alternifolia leaf section offers a detailed view of the cellular structures, pigments, and chloroplast morphology, allowing for a more in-depth understanding of the plant's photosynthetic and physiological characteristics:
Pigmentation
The cells within the leaf section display a vibrant array of pigments, primarily chlorophyll, which is responsible for the green coloration observed. Additionally, there are hints of reddish and yellowish pigments, likely carotenoids or anthocyanins, dispersed throughout the cells. The distribution of these pigments is somewhat uniform, with each cell containing multiple pigment granules. This uniformity suggests efficient light absorption and a well-adapted mechanism for photosynthesis, even in varying light conditions typically encountered in an aquatic environment.
Chloroplast Morphology
The chloroplasts within the cells are clearly visible, characterized by their distinct, oval to rounded shapes. The size of the chloroplasts appears relatively consistent across different cells, with each cell containing numerous chloroplasts, evenly distributed along the cell walls. The morphology of these chloroplasts indicates a high degree of specialization for capturing light energy, with their arrangement optimizing the surface area exposed to light within each cell. The presence of numerous chloroplasts per cell highlights the plant’s adaptation to its aquatic environment, where light availability can fluctuate.
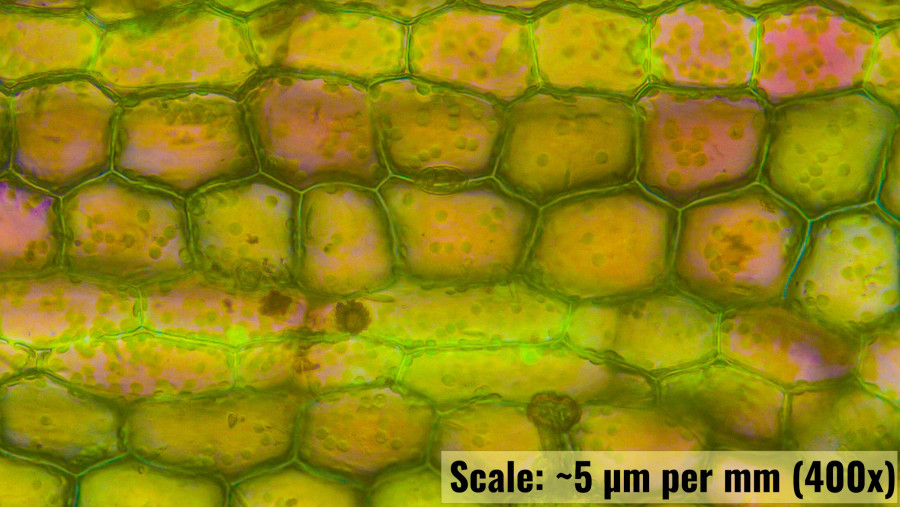
Cellular Arrangement
The cells are hexagonal and tightly packed, forming a robust and interconnected network. This close packing not only provides structural integrity to the leaf but also maximizes the efficiency of light capture and photosynthetic activity. The absence of large intercellular spaces suggests that the leaf is designed to minimize water loss and maintain buoyancy within its aquatic habitat.
Pigment Granules
Upon close observation, pigment granules within the chloroplasts are noticeable, possibly representing starch grains or other photosynthetic by-products. These granules appear as small, dark dots within the chloroplasts, indicating active photosynthesis and the storage of photosynthetic products. The granules are more concentrated near the cell walls, suggesting that these areas might be involved in the transport or storage of energy-rich compounds produced during photosynthesis.
Magnification Estimate
Given the clarity and detail of individual chloroplasts, as well as the visibility of pigment granules within these organelles, the magnification is likely in the range of 400x to 1000x. This level of magnification allows for a comprehensive view of the internal structure of the cells, including the fine details of the chloroplasts and pigment granules.
The high-magnification observation of the N. alternifolia leaf section reveals a highly organized cellular structure, with chloroplasts and pigments efficiently arranged to optimize photosynthetic activity. The uniform distribution of chloroplasts, coupled with the presence of pigment granules, underscores the plant’s adaptation to its aquatic environment, where light conditions can vary. The detailed view provided by the high magnification (estimated at 400x to 1000x) offers valuable insights into the physiological strategies employed by N. alternifolia to thrive in its unique habitat.
Comparative Observation between N. alternifolia to H. verticillata Leaf Histology
The histological analysis of N. alternifolia using a compound light microscope and photography system revealed a highly primitive and almost unicellular architecture, allowing for direct wet mounts to be prepared (Figs. 1-3). This characteristic is like that of H. verticillata, a submerged aquatic plant that also exhibits a simple cellular structure (Fig. 4) (Cook & Urmi-König, 1985). In fact, both species have been reported to have a reduced cellular complexity, likely an adaptation to their aquatic environments (Sculthorpe, 1967).
The simplicity of the cellular structure in N. alternifolia is evident in the lack of differentiated tissues, such as xylem and phloem, absent Sclerenchyma, which are typically found in more complex plant species (Esau, 1977a). Similarly, H. verticillata has been shown to lack these tissues, with water and nutrient transport occurring through a network of parenchymatous cells (Cook & Urmi-König, 1985).
However, there are some notable differences in the histology of the two species. For example, N. alternifolia exhibits a higher degree of cellular vacuolization, which may be an adaptation to its environment (Figs. 3-4). In contrast, H. verticillata has been reported to have smaller, more numerous vacuoles (Cook & Urmi-König, 1985).
H. verticillata exhibited a well-defined leaf edge with a prominent, curved thorn-like projection. The cellular structure appeared organized, with a clear epidermis and underlying tissue visible. N. alternifolia presented a markedly different appearance, with a seemingly disorganized cellular structure. The tissue showed a mix of green and pink coloration, with multiple irregular protrusions along the edge. Notably, distinct vascular tissues such as phloem were not clearly discernible, suggesting a less organized internal structure compared to the other specimens (Bowes, G., Holaday, A. S., et al 1979).
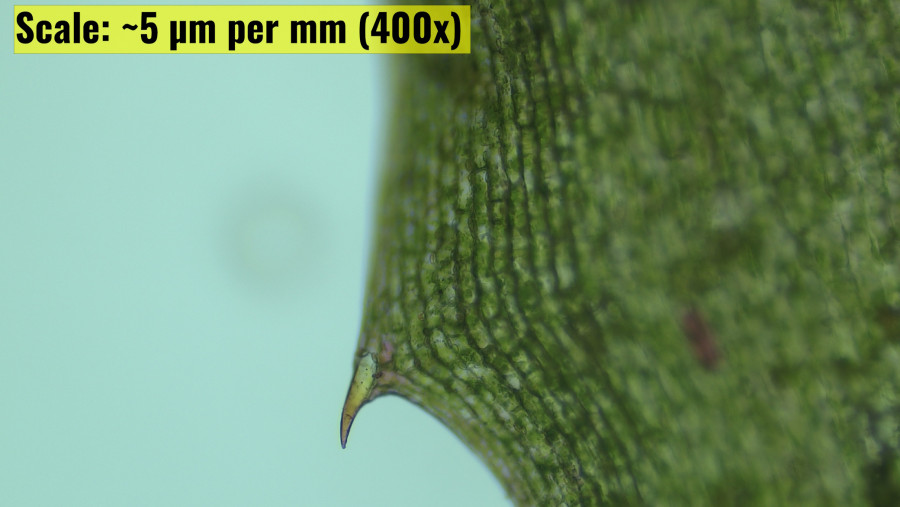
Both N. alternifolia and H. verticillata are well-adapted to their aquatic environments, but they exhibit distinct histological differences that reflect their unique ecological niches. Nechamandra has thicker, more robust leaves with tightly packed hexagonal cells and diverse pigmentation, allowing it to thrive in a variety of light conditions and water movements. In contrast, Hydrilla has thinner, more flexible leaves with elongated cells and a uniform green coloration, reflecting its specialization in low-light, stable water environments. The differences in leaf margin structure and cellular organization further highlight the distinct adaptive strategies employed by these two aquatic plants
Comparative Observation between N. alternifolia and N. indica Leaf Histology
Cellular Arrangement
The leaf cells of Najas are often more elongated and arranged in a more linear fashion compared to the hexagonal cells in Nechamandra. Najas leaves typically exhibit a single layer of elongated epidermal cells, which are less compact than those observed in Nechamandra (Chamisso, A. & Schlechtendal, D.F.L. 1826).

Chloroplast Distribution
In Najas, chloroplasts are also abundant but may be more concentrated near the periphery of the cells. The chloroplasts in Najas are generally smaller and less densely packed than those in Nechamandra, which may be a reflection of different light environments or photosynthetic strategies (Ruzin, S. E. 1999).
Pigmentation
Najas typically shows a more uniform green pigmentation dominated by chlorophyll, with less variation in other pigments. This may indicate a more specialized photosynthetic adaptation to stable, low-light conditions typically found in submerged environments (Ruzin, S. E. 1999).
Cell Shape and Size
Cells in Najas tend to be elongated and less densely packed, with a thinner cell wall compared to Nechamandra. This might make Najas leaves more flexible and less rigid, an adaptation to the flowing water environments where Najas is often found.
Leaf Margins
Najas typically has smooth or finely serrated leaf margins, with less pronounced teeth compared to Nechamandra. This difference in leaf margin structure may reflect different ecological strategies or mechanical adaptations between the two genera (Ruzin, S. E. 1999).
While both N. alternifolia and N. indica species are adapted to aquatic environments, they exhibit distinct differences in leaf histology. Nechamandra has more tightly packed, hexagonal cells with diverse pigmentation and larger, more numerous chloroplasts, which likely contribute to its adaptability in varying light conditions. In contrast, Najas species have more elongated, less densely packed cells with a more uniform chloroplast distribution, reflecting their specialization in stable, low-light aquatic habitats. These differences highlight the unique adaptations of each genus to their respective environmental niches (Ruzin, S. E. 1999).
Cytoplasmic Streaming or Cyclosis in N. alternifolia
Cytoplasmic streaming, or cyclosis, plays a crucial role in the cellular function of aquatic plants, aiding in the efficient transport of nutrients, organelles, and other essential materials within the cell. This process is particularly significant in large vacuolated cells of aquatic plants, where the movement of cytoplasm helps in distributing chloroplasts more evenly, optimizing photosynthesis under varying light conditions (Shimmen & Yokota, 2004). In species like Chara, Nitella, and Nechamandra, cytoplasmic streaming facilitates the rapid movement of materials across extensive cell lengths, thereby maintaining cellular homeostasis and contributing to the overall growth and adaptation of the plant in aquatic environments (Kamiya, 1981).
Discussion
Importance of Studying N. alternifolia: Highlighting Its Potential Invasive Nature
N. alternifolia, a lesser-known aquatic plant, presents a unique combination of enigmatic characteristics and ecological significance. As an understudied species, N. alternifolia is of particular interest due to its potential to become an invasive species in non-native ecosystems. The study of this plant is crucial for several reasons, primarily focusing on its ecological impact, adaptability, and the potential consequences of its spread.
One of the primary reasons for studying N. alternifolia is to understand its ecological role in its native habitat and how this might change if it spreads to new environments. In its natural setting, N. alternifolia may contribute positively to the ecosystem by providing habitat for aquatic organisms, stabilizing sediments, and contributing to nutrient cycling. However, when introduced to non-native environments, the same traits that make it successful in its natural habitat could lead to dominance over local flora, resulting in significant ecological disruptions. The plant’s ability to grow rapidly and form dense mats can outcompete native species for light, space, and nutrients, leading to a decrease in biodiversity (Simberloff, 2000).
The potential invasive nature of N. alternifolia is particularly concerning given its adaptability to various aquatic environments. The plant’s resilience to different water conditions, such as varying levels of light, nutrients, and water depth, could allow it to establish itself in a wide range of habitats. This adaptability, while beneficial in its native range, could pose a serious threat to non-native ecosystems if the plant were to be introduced, either intentionally or accidentally, through human activities such as aquarium trade, water gardening, or transportation via watercraft (Richardson & Rejmánek, 2011).
Moreover, the study of N. alternifolia is essential for the development of effective management and control strategies. Understanding the plant's biology, growth patterns, and reproductive strategies can inform approaches to prevent its spread and mitigate its impact if it becomes established in new areas. Early detection and rapid response are critical components in managing invasive species, and thorough knowledge of N. alternifolia will be vital in these efforts (Mack et al, 2000).
The enigmatic nature of N. alternifolia coupled with its potential invasive tendencies makes it a critical subject of study. By investigating its ecological role, adaptability, and possible impacts on non-native environments, researchers can better predict and manage the risks associated with this species. Proactive research and monitoring are essential to prevent N. alternifolia from becoming a threat to biodiversity and ecosystem stability in regions where it does not naturally occur.
Implications for Management Strategies
Understanding the fine details of N. alternifolia through advanced imagery offers numerous benefits in developing tailored management approaches. Firstly, early detection allows timely implementation of eradication efforts aimed at preventing large-scale infestations (Thiébaut et al, 2010). Secondly, accurate discrimination enables informed decision-making concerning suitable biological controls without compromising indigenous ecosystem integrity (Anderson et al, 2004). Lastly, intensified surveillance programs backed by sound science facilitate responsible trading practices, safeguarding fragile environments from accidental introductions or deliberate translocations.
Moreover, given the increasing importance of N. alternifolia in the aquarium industry via internet trade, allowing it to reach the four corners of the world, knowing its microscopic anatomy could assist in optimizing growth parameters, improving cultivation practices, and enhancing aesthetic appeal. In summary, pursuing a histological or microscopic anatomy study focused on N. alternifolia holds significant promise for advancing both scientific curiosity and practical application.
While previous investigations concentrate on N. alternifolia's broader facets, the pressing issue lies in scrutinizing its finer structural dimensions. Examining the light microscopic anatomy of wet N. alternifolia specimens constitutes a critical step towards addressing mounting concerns over its probable invasive tendencies. Drawing lessons from the history of H. verticillata's proliferation, prompt action targeting N. alternifolia warrants serious consideration by policymakers, scientists, and industry professionals alike.
Conclusions
The histological examination of N. alternifolia through wet mount light microscopy has provided significant insights into the plant's structural adaptations to its aquatic environment. The study reveals several key anatomical features that facilitate Nechamandra’s survival and functionality in submerged conditions. These include the development of large intercellular spaces (aerenchyma) for buoyancy and gas exchange, a thin epidermis without a cuticle for efficient nutrient and water absorption, and a simplified vascular system adapted to the plant's aquatic habitat.
These findings underscore the importance of anatomical specialization in aquatic plants, demonstrating how N. alternifolia has evolved specific features to thrive in water.
The comparative approach highlights the differences between N. alternifolia and terrestrial plants, particularly in terms of structural complexity and resource management strategies.
This research contributes to the broader understanding of plant adaptation in aquatic environments and offers a foundation for future studies on the evolutionary biology of submerged macrophytes. Through this work, the intricate relationship between form and function in aquatic plants becomes clearer, emphasizing the role of environmental pressures in shaping plant anatomy.
Comparative analysis with terrestrial plants and other aquatic species such as Hydrilla verticillata and Najas indica underscores the specialized nature of N. alternifolia's cellular organization. These adaptations demonstrate the plant's evolutionary response to the challenges of an aquatic lifestyle.
This research contributes to our understanding of plant adaptation in aquatic environments and provides a foundation for future studies on the evolutionary biology of submerged macrophytes. It also highlights the importance of microscopic anatomy in plant taxonomy and ecological studies, particularly for species that may become invasive in non-native habitats. Further investigations should focus on the physiological implications of these structural characteristics, particularly in relation to gas exchange, nutrient storage, and the plant's potential invasive behavior in new ecosystems.
Authors contributions
SKR: Conceptualization, Writing; RSI: Data analysis, Review; SV: Investigation, Editing
Acknowledgments
None
Conflict of interest
No conflict of interest.
References
American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 23rd ed. Washington, DC: American Public Health Association, 2017.
Anderson, J. M., S. P. Harrison, and B. Bowes. "Differences in Gas Exchange Characteristics between Submerged Macrophyes." Aquatic Botany 32, no. 2 (1988): 149–161. https://doi.org/10.1016/0304-3770(88)90048-1.
Anderson, L. W. J., J. L. Gallagher, E. D. Grosholz, E. H. Heugens, D. Simberloff, M. Williamson, and D. W. Wolfe. "Review of Integrated Population Models Applied to Alien Species Management." Conservation Biology 18, no. 3 (2004): 706–718. https://doi.org/10.1111/j.1523-1739.2004.00607.x.
Bellinger, P. F., S. J. Baird, and G. D. Britton. Algae: Anatomy, Biochemistry, and Biotechnology. Cambridge: Cambridge University Press, 2019.
Bowes, G., and M. E. Salvucci. "Plasticity in the Photosynthetic Carbon Metabolism of Submerged Aquatic Macrophytes." Aquatic Botany 34, no. 1 (1989): 233–266. https://doi.org/10.1016/0304-3770(89)90058-7.
Bowes, G., A. S. Salvucci, and W. T. Haller. "Seasonal Variation in the Biomass, Tuber Density, and Photosynthetic Metabolism of Hydrilla in Three Florida Lakes." Journal of Aquatic Plant Management 17, no. 1 (1979): 61–65.
Brix, H. "Plant Biomass Production, Decomposition and Mineralization Rates in Submerged Lakes." Aquatic Botany 57, no. 1 (1997): 1–26.
Cook, C. D. K. An Introduction to Aquatic Plants. Oxford: Blackwell Science, 1996.
Cook, C. D. K. Aquatic and Wetland Plants of India. New York: Oxford University Press, 1996.
Cook, C. D. K. and K. Ur. König. "A Revision of the Genus Hydrilla (Hydrocharitaceae)." Aquatic Botany 21, no. 4 (1985): 285–289. https://doi.org/10.1016/0304-3770(85)90072-7.
Dr. Santhosh Kumar Rajamani.. Histology of Nechamandra Alternifolia. . Available from: https://github.com/kealian/histology-of-Nechamandra, 2024.
Esau, K. Anatomy of Seed Plants, 2nd ed. New York: John Wiley & Sons, 1977.
Esau, K. Plant Anatomy: Meristems, Cells, and the Plant Body. Enlarged New Edition. New York: John Wiley & Sons Inc., 1977.
Geng, Y., S. Wang, X. Liang, L. Zhang, J. Yang, M. Cheng, et al. "Invasions of Hydrilla through Bottom–Up Cascading Effects." Ecology Letters 21, no. 11 (2018): 1433–1443.
Gessner, F. O. Aquarium Plants: Biology and Culture. Neptune City, NJ: T.F.H. Publications, 1957.
Graham, L., L. W. Wilcox, and J. M. Graham. Alga. Upper Saddle River, NJ: Prentice Hall, 2000.
Gunawardena, N. R. E. T., L. V. I. D. S. Madurwarige, K. N. U. Jayasekara, A. A. U. Gunathilaka, W. D. O. N. Amarasinghe, and G. Seneviratne. "Comparative Analysis of Veination Patterns among Some Selected Hydrocharitaceous Taxa." Journal of Applied Botany and Food Quality 88, no. 1 (2015): 57–61. https://doi.org/10.5073/JABFQ.2015.088.014.
Hussain, F., M. J. Durrani, and G. Rehman. "Threatened and Rare Aquatics of Bannu Basin (Pakistan)." Journal of Biological Sciences 10, no. 7 (2010): 618–623.
Hussner, L., L. Bergström, J. Bastmeijer, K. Brock, A. W. Damman, E. Van Donk, and E. Blindow. "Submersed Macrophytes in Europe: Status, Trends, Perspectives, and Conservation." Hydrobiology 716, no. 1 (2013): 1–14. https://doi.org/10.1007/s10750-012-1404-x.
Jaiswal, S., and M. Sriastava. "Genetic Diversity of Nechamandara Alternifolia using RAPD and ISSR Markers." Journal of Aquatic Plant Management 53, no. 2 (2015): 85–92.
Kamiya, N. "Physical and Chemical Basis of Cytoplasmic Streaming." *Annual Review of Plant Physiology* 32, no. 1 (1981): 205–236.
Kapoor, L. D. *Handbook of Ayurvedic Medicinal Plants: Herbal Reference Library.* Boca Raton, FL: CRC Press, 1986.
Kenrick, P., and P. R. Crane. "The Origin and Early Evolution of Plants on Land." *Nature* 389, no. 6646 (1997): 33–39. https://doi.org/10.1038/389033a0.
Kozlowski, T. T., and S. G. Pallardy. *Physiology of Woody Plants.* San Diego, CA: Academic Press, 2019.
Kundu, S., S. Bhattacharya, and A. Ghosh. *Aquatic Plant Diversity and Conservation in India.* Singapore: Springer, 2018.
Köhler, A. "Über die Konstruktion und Berechnung der Mikroskopischen Beleuchtungsapparate." *Jenaische Zeitschrift für Naturwissenschaften* 26, no. 1 (1893): 1–42.
Les, D. H., M. A. Cleland, and M. Waycott. "Phylogenetic Studies in the Monocotyledons: Alismatidae." *Aliso: A Journal of Systematic and Evolutionary Botany* 22, no. 1 (2006): 1–12.
Les, D. H., M. L. Moody, S. W. L. Jacobs, and R. J. Bayer. "Systematics of Two Imperiled Aquatic Genera (Hydrilla and Najas, Hydrocharitaceae)." *Systematic Botany* 33, no. 2 (2008): 277–283. https://doi.org/10.1600/036364408784571547.
Ligrone, R., A. Carafa, and J. G. Duckett. "The Evolution of the Bryophytes: A Phylogenetic Perspective." *Plant Systematics and Evolution* 298, no. 1 (2012): 1–19. https://doi.org/10.1007/s00606-011-0485-6.
Liu, J., Q. Liang, C. Wang, P. Yang, Y. Huang, and X. Cheng. "Comparison of Leaf Venation Pattern and Stomatal Characteristics between Floating-Leaved and Emerged Macrophytes in Lake Taihu." *Ecological Engineering* 136 (2019): 249–257. https://doi.org/10.1016/j.ecoleng.2019.03.031.
Liu, Q., H. Fang, L. Sun, L. Song, T. Luo, Y. Wu, and Z. Yu. "Morphological Characteristics and Genetic Diversity Analysis of Six Chinese Endemic Aquatic Plants Using Internal Transcribed Spacer Sequences." *Aquatic Plants* 15, no. 1 (2019): 1–11. http://dx.doi.org/10.1515/aquap-2019-0001.
Lodge, D. M. "Herbivory on Freshwater Macrophytes." *Aquatic Botany* 41, nos. 1–3 (1991): 195–224. https://doi.org/10.1016/0304-3770(91)90042-6.
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Evans, M. Clout, and F. A. Bazzaz. "Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control." *Ecological Applications* 10, no. 3 (2000): 689–710. https://doi.org/10.1890/1051-0761(2000)0102.0.CO;2.
Madsen, J. D., N. Oezguen, L. W. J. Anderson, T. D. Center, J. Grimsby, A. J. Hanson, et al. "Identifying *Hydrilla Verticillata* (L. f.) Royle and Distinguishing It from Other Submerged Aquatic Plants." Technical Report No. 15–02. USDA National Wildlife Research Center, 2015. Retrieved from https://www.nrcs.usda.gov/.
Menzel, C., and J. Sharry. "Anatomical Variation among Shoots of *Elodea Canadensis* Michx. (Hydrocharitaceae) along an Urban–Rural Gradient." *Aquatic Botany* 116 (2014): 35–42.
Newman, R. M. "Herbivory and Detritivory on Freshwater Macrophytes by Invertebrates: A Review." *Journal of the North American Benthological Society* 10, no. 2 (1991): 89–114. https://doi.org/10.2307/1467571.
Pedersen, O., K. Sand‐Jensen, T. Binzer, and T. B. Christensen. "Functional Traits Explain Distribution Patterns in European Seagrass Meadows." *Journal of Ecology* 102, no. 2 (2014): 400–411. https://doi.org/10.1111/1365-2745.12243.
Persoon, C. H. *Ottelia Alismoides* (L.) Pers., *Species Plantarum* (4th ed., Vol. 2, p. 481). Berlin: Sumptibus Guilielmi, 1806.
Rai, S. K., A. Kumar, P. Sharma, and D. Singh. "Morphological and Ultrastructural Characterization of *Hydrilla Verticillata* (L.f.) Royle under Different Environmental Conditions." *Journal of Applied Phycology* 29, no. 1 (2017): 401–410.
Raja, R. R., P. Ramachandran, K. Anantha, G. Venkataraman, P. Balaji, P. Arulselvan, et al. "Ethnobotanical Survey of Aquatic Plants Used in Traditional Medicine among Paliyar Tribes of Western Ghats, South India." *International Journal of Advanced Research* 3, no. 6 (2015): 1351–1360.
Rao, P. S. N. 2016. Aquatic Biodiversity in India: The Neglected and the Unexplored. World Aquatic Biodiversity.
Raven, Peter H., Ray F. Evert, and Susan E. Eichhorn. 2005. Biology of Plants. 7th ed. New York: W.H. Freeman and Company.
Richardson, David M., and Marcel Rejmánek. 2011. “Trees and Shrubs as Invasive Alien Species: A Global Review.” Diversity and Distributions 17 (5): 788–809. https://doi.org/10.1111/j.1472-4642.2011.00782.x.
Royle, J. F. 1839. Hydrilla Verticillata (L.f.) Royle.
Ruzin, Steven E. 1999. Plant Microtechnique and Microscopy. New York: Oxford University Press.
Sculthorpe, C. D. 1967. The Biology of Aquatic Vascular Plants. London: Edward Arnold Ltd.
———. 1985. The Biology of Aquatic Vascular Plants. London: Edward Arnold Ltd.
———. 2003. The Biology of Aquatic Vascular Plants. New York: Oxford University Press.
Shimmen, T., and E. Yokota. 2004. “Cytoplasmic Streaming in Plants.” Current Opinion in Cell Biology 16 (1): 68–72.
Simberloff, Daniel. 2000. “Nonindigenous Species: A Global Threat to Biodiversity and Stability.” In Nature and Human Society: The Quest for a Sustainable World, edited by Peter Raven, 325–34. Washington, DC: National Academies Press.
Thiébaut, Eric, Miguel Clavero, Johannes Englund, Pietro Genovesi, J. M. Jeschke, Robert P. Keller, et al. 2010. “Early Detection Surveys for Exotic Plant Species: Challenges and Solutions.” Journal of Environmental Management 91 (2): 772–80. https://doi.org/10.1016/j.jenvman.2009.12.004.
Thwaites, G. H. K. 1861. Nechamandra Alternifolia (Roxb.) Thwaites.
Touchette, Brian W., and JoAnn M. Burkholder. 2000. “Review of Nitrogen and Phosphorus Metabolism in Seagrasses.” Journal of Experimental Marine Biology and Ecology 250 (1–2): 133–67. https://doi.org/10.1016/S0022-0981(00)00195-7.
Triest, Ludwig. 1988. “Genetic Variation and Differentiation of Najas Marina ssp. Armata (Hydrocharitaceae) in Belgium and Northern France.” Aquatic Botany 30 (1–2): 155–62. https://doi.org/10.1016/0304-3770(88)90084-1.
Westlake, D. F. 1967. “Some Effects of Low-Velocity Currents on the Metabolism of Aquatic Macrophytes.” Journal of Experimental Botany 18 (3): 187–205. https://doi.org/10.1093/jxb/18.3.187.
Zhang, Ying, Lei Wang, Xin Li, Hao Sun, Wenxin Chen, and Qiang Huang. 2019. “Comparative Morpho-Anatomical Investigation of Five Submerged Macrophytes in China’s Largest Freshwater Lake.” Aquatic Botany 157: 122–30.
Zhao, Feng, Guanghe Tian, Min He, Yanyan Yang, Bin Yan, and Qiang Wu. 2018. “Chloroplast Genome Evolution in Cabomba Caroliniana (Cabombaceae): Insights from Complete Plastome Sequences.” Molecular Phylogenetics and Evolution 120: 115–23.
Ziegler, H. 1961. “Wasserleitungsbahnen und Transfusionsgewebe bei Monocotylemen.” Planta 56 (1): 73–94. https://doi.org/10.1007/BF01922351.





